Table of Contents:
- Challenges Faced by Importers & Exporters Shipping Coffee to the US
- Requirements for Importing Coffee into the U.S.A.
- Importing Coffee into the U.S: FDA Compliance
- FDA Prior Notice Filing
- Food Labelling Compliance
- Food Facility & DUNS Registration
- FSVP Compliance
- How CrimsonLogic can Help you Import Coffee into the U.S.
- What Makes CrimsonLogic FDA Managed Services Better than Others?
Coffee is one of the most popular beverages in the world. The United States has the biggest coffee market, with worldwide revenues of more than 80 billion U.S. dollars in 2021. Colombia and Brazil, on the other hand, are the leading exporters to the U.S., exporting over one billion dollars worth of coffee in the most recent year.
Can you imagine a day without coffee? In the U.S., as with the rest of the world, millions upon millions of consumers start their day with a hot cup of coffee and then consume more of it throughout the day. The coffee segment is expected to grow about +1.9% next year.
The demand for specialty coffee showed an +8.0% year-on-year growth and there is a growing diversity in tastes, driving opportunities for different kinds of coffee and different kinds of consumption. The burgeoning demand for coffee is encouraging more café players and coffee producers to enter the market. Clearly, there’s never been a better time to be in this trade.
However, for importers and other participants, a key challenge is successfully navigating the shipping and logistics of importing coffee from other countries into the US. This means maintaining compliance with FDA regulations while establishing a highly efficient and cost-effective process. In this article, we’ll discuss how to import coffee into the U.S. and several shipping and logistics options.
Challenges Faced by Importers & Exporters in Shipping Coffee to the US
As a global importer or exporter, shipping and logistics can be a huge challenge, particularly if you are new to the process. You may not have the knowledge and expertise, and the infrastructure to manage the process from locations across the globe. These can result in failure to achieve 100% compliance with FDA regulations, which can lead to delays and the extra cost of transporting the products back to the country of origin.
Time is of the essence when it comes to coffee. Coffee should be as fresh as possible, and delays will further shorten the window to sell. Delays can cause quality issues such as:
- Undesirable flavor and aroma – older beans become more porous and tend to leave a rancid or musty aroma and hence, an undesirable flavor on the palate
- Loss of coffee fragrance – while coffee beans do not smell bad over time, they do lose their fragrance
These quality issues could affect your coffee’s ability to make its mark as specialty coffee, affecting your ability to sell and damaging your brand over time.
From a supply chain and logistics management standpoint, many importers and exporters shipping coffee to the U.S. are having a hard time finding a reliable and experienced trade compliance partner who can provide 24/7 multi-language dedicated support. This is a crucial component that can help avoid additional costs due to delays and ensure a hassle-free entry of products into the U.S.
Requirements for Importing Coffee into the U.S.A.
To import coffee into the U.S., you must meet the requirements of three U.S. government agencies: the U. S. Customs and Border Protection (CBP), Food and Drug Administration (FDA), and the United States Department of Agriculture (USDA).
To have your coffee products cleared smoothly, you, as an importer, need to comply with CBP policies and procedures, before actually importing them. This process can be complicated and time-consuming for many importers. In this case, your best option would be to work with a trade compliance partner who offers reliable cross-border trade and clearance solutions across all channels.
Coffee, like all other food products, is subject to review by the FDA before it is granted entry into the U.S. The FDA electronically reviews all FDA-regulated shipments submitted to the CBP. Food products must comply with all applicable FDA regulations or be refused entry. The importer is held responsible for ensuring this. First-time importers and those who do not have the in-house knowledge, time, experience and infrastructure should consider a service like the FDA Managed Services that can help them navigate the constantly-changing U.S. regulatory environment.
Importing Coffee into the U.S: FDA Compliance
FDA compliance for importing coffee to the U.S. typically involves 4 items: FDA Prior Notice Filing, Food Labelling Compliance, Food Facility & DUNS Registration and FSVP Compliance.
FDA Prior Notice Filing
Coffee importers must send FDA Prior Notice before coffee is shipped for import into the U.S. The FDA Prior Notice is an electronic notification that all importers or their authorized agents must submit to the FDA before any shipment containing feed or food products arrives in the U.S. The FDA must receive and validate the pre-arrival Prior Notice, all within the mode-specific timeframes.
Food shipments that do not meet the FDA Prior Notice requirements may be rejected, abandoned or destroyed. The importer may also receive criminal and financial penalties.
Food Labelling Compliance
Importers must ensure that their coffee labels comply with FDA regulations. If the products are not properly labelled, they will not be allowed to enter the U.S.
Important details to include on the label:
- Name of food/product
- Ingredients
- Country of origin for the product
- English language labelling
- Nutritional information
- Any chemicals/food additives used
- Food allergens
Food Facility & DUNS Registration
Importers must register all facilities that manufacture, pack, process, receive or hold their coffee products that are offered for consumption in the US. They must renew this registration every two years.
FSVP Compliance
Under the Foreign Supplier Verification Program (FSVP) for the FDA, importers must perform risk-based foreign supplier verification.
How CrimsonLogic Can Help You Import Coffee into the U.S.
You can choose to either manage the FDA compliance work yourself or outsource it to a compliance agent. To help you decide which is better for your business, let’s look at the differences:
Still not sure whether to DIY or outsource? Need more advice for your business?
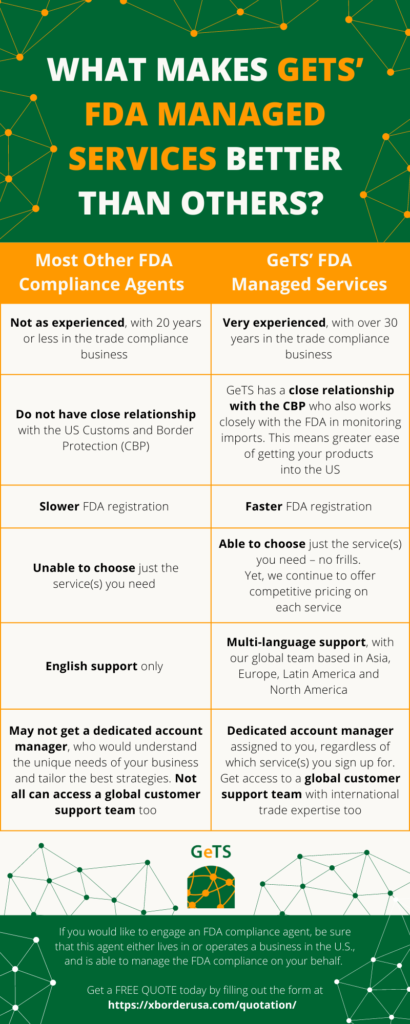
What Makes CrimsonLogic FDA Managed Services Better than Others?
If you would like to engage an FDA compliance agent, be sure that this agent either lives in or operates a business in the US, and is able to manage the FDA on your behalf.
Visit our FDA Managed services page or contact us today to learn more.